Carbapenem-Resistant Organisms (CROs)
The Alameda County Health Officer Order dated June 13, 2017, requiring health care providers and clinical laboratories to report all cases of carbapenem-Resistant Enterobacterales (CRE) to Alameda County Public Health Department (ACPHD) and submit associated specimens to Alameda County Public Health Laboratory (ACPHL) is hereby rescinded and replaced with this order, effective November 3, 2025:
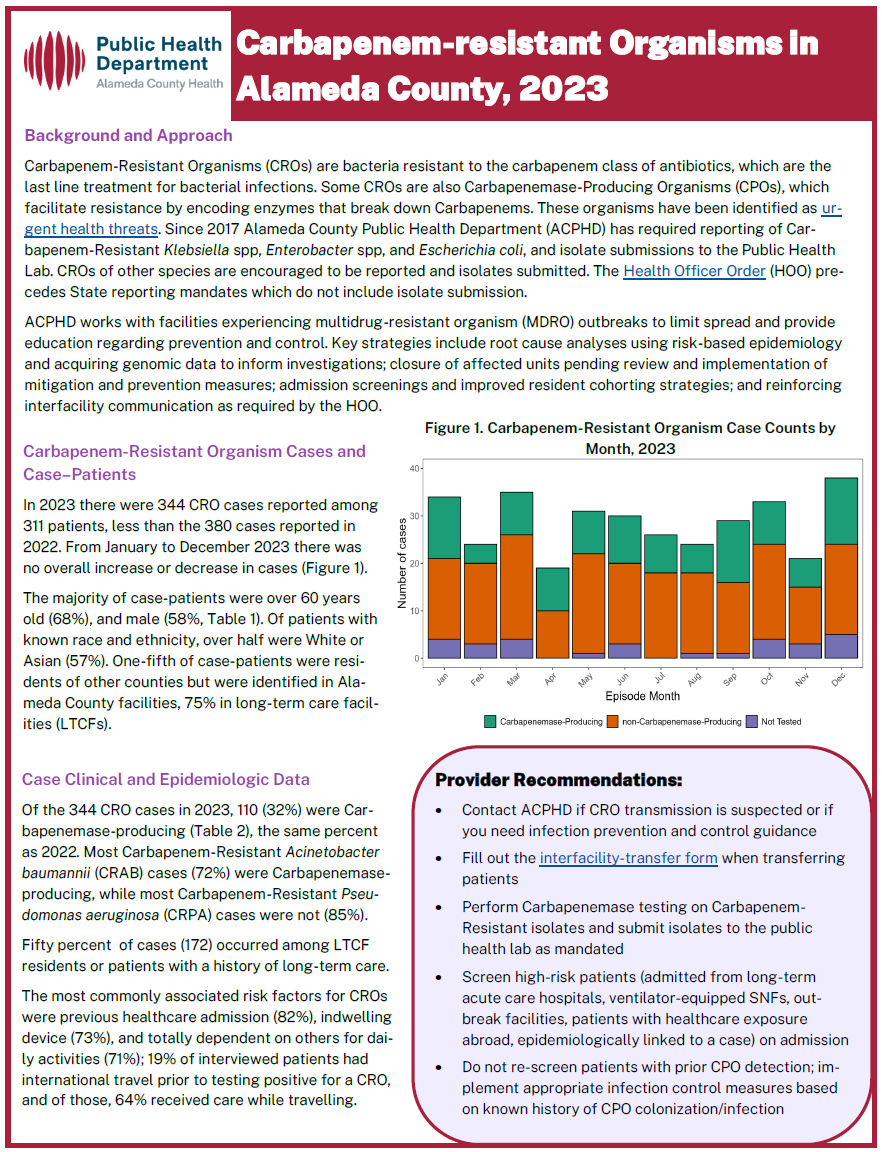
Providers must report CRO cases meeting the following criteria to ACPHD:
- For CRE: resistance to any carbapenem antimicrobial, with a MIC of ≥ 4 µg/ml for doripenem, imipenem, or meropenem; or MIC ≥ 2 µg/ml for ertapenem; OR
- For carbapenem-resistant Acinetobacter Baumannii (CRAB): resistance to any carbapenem antimicrobial, with a MIC of ≥ 8 µg/ml for doripenem, imipenem, or meropenem OR
- For carbapenem-resistant Pseudomonas aeruginosa (CRPA): resistance to any carbapenem antimicrobial, with a MIC of ≥ 8 µg/ml for doripenem, imipenem, or meropenem AND nonsusceptible (intermediate or resistant MIC ≥ 16 µg/ml) to cefepime or ceftazidime, or, resistant to ceftolozane/tazobactam (MIC ≥ 16/4μg/ml); OR
- For any CRO: Documented carbapenemase production, demonstrated using a phenotypic CLSI-endorsed or FDA cleared test (modified Hodge, Carba-NP, metallo-β-lactamase, etc.); OR
- For any CRO: demonstrated to possess a carbapenemase gene (such as KPC, NDM, VIM, IMP, OXA-48-type) using a CLSI-endorsed or FDA cleared test (Carba-R, PCR, Whole Genome Sequencing, etc.)
Laboratories must submit CRO microbiologic isolates meeting the following criteria to ACPHL:
- The isolate is CRAB, regardless of carbapenemase test results OR
- The isolate has not already undergone carbapenemase testing at a facility or commercial laboratory OR
- The isolate had a positive phenotypic test, but a specific carbapenemase was not identified OR
- The isolate is pan-nonsusceptible (intermediate or resistant to all drugs tested to date) OR
- The isolate is specifically requested by the ACPHD OR
- The isolate belongs to an Alameda County resident residing in a LTCF or SNF
 Carbapenem-resistant organisms, or CROs, are a type of multidrug-resistant (MDRO) organism that can cause both infection and colonization in healthcare settings. CROs include carbapenem-resistant Acinetobacter baumannii (CRAB) and carbapenem-resistant Pseudomonas aeruginosa (CRPA) both of which have been identified in Alameda County. When Enterobacterales, a group of Gram-negative, non-spore forming bacteria, develop resistance to carbapenems, they are referred to as carbapenem-resistant Enterobacterales (CRE). In 2017 there were an estimated 13,100 hospitalizations and 1,100 deaths due to CREs in the U.S.
Carbapenem-resistant organisms, or CROs, are a type of multidrug-resistant (MDRO) organism that can cause both infection and colonization in healthcare settings. CROs include carbapenem-resistant Acinetobacter baumannii (CRAB) and carbapenem-resistant Pseudomonas aeruginosa (CRPA) both of which have been identified in Alameda County. When Enterobacterales, a group of Gram-negative, non-spore forming bacteria, develop resistance to carbapenems, they are referred to as carbapenem-resistant Enterobacterales (CRE). In 2017 there were an estimated 13,100 hospitalizations and 1,100 deaths due to CREs in the U.S.
The 2019 CDC Antibiotic Resistance Threats Report lists CROs as Urgent Threats, which is the highest level of concern. Since the COVID-19 pandemic, the threat of antimicrobial resistance has only become more dire, with hospital-acquired antibiotic-resistant infections increasing by at least 15% in the first year of the pandemic. There are many reasons for this increase in antibiotic resistance including changed healthcare-seeking behaviors allowing undiagnosed and untreated infections to spread, increasing chances of developing resistance; from March to October of 2020, more than 80% of patients hospitalized with COVID-19 received unnecessary antibiotic therapy due to difficulty of distinguishing COVID-19 pneumonia from community-acquired pneumonia, increasing ability of bacteria to develop resistance.
CROs can be classified by their mechanism of resistance, non-carbapenemase producing organisms (non-CP), and carbapenemase-producing organisms (CPO). Carbapenemases are enzymes that break down antibiotics and render them ineffective. These enzymes are categorized into Class A, B, and D. Some common carbapenemases are Klebsiella pneumoniae carbapenemase (KPC), imipenamase (IMP), Verona integron-encoded metallo-β-lactamase (VIM), New Delhi metallo-β-lactamase (NDM), and oxacillinase (OXA). CPOs are of special public health concern because of their ability to easily spread resistance between many species of bacteria in healthcare settings.
Risk factors for CRO colonization or infection include prolonged inpatient stays, severe illness and/or comorbid conditions, invasive medical devices (such as catheters, endotracheal tubes, and feeding tubes), and treatment with certain antibiotics. Because patient populations in long-term care facilities (LTCF) and skilled nursing facilities (SNF) tend to have many of these risk factors, healthcare teams in these facilities should be especially cognizant of CROs.
The spread of CROs is mediated by patient transfer between facilities and inconsistently applied infection control measures. This highlights the importance of using the Infection Control Transfer Form and ensuring your facility is prepared for a patient with CRO, such as implementing appropriate infection control practices and environmental cleaning. In addition, California Department of Public Health (CDPH) advocates that all residents of SNFs be on Enhanced Standard Precautions which is a resident-centered activity-based approach to help limit the spread of resistant organisms.
On June 13, 2017, Alameda County Public Health Department (ACPHD) issued a Health Officer Order requiring providers and clinical laboratories to report carbapenem-resistant Enterobacterales (CRE) cases, which are a subset of CROs, and requiring clinical laboratories to submit clinical isolates of CREs. Since then, CRO surveillance needs have evolved due to increases in cases and advances in identification of carbapenemase genes at healthcare facilities. While the California Department of Public Health (CDPH) mandated laboratory reporting of carbapenemase-producing organisms in 2022 (California Code of Regulations, Title 17, Section 2505), there is still a CPO surveillance gap in Alameda County as not all healthcare facilities can test for carbapenemase genes. This updated Health Officer Order goes beyond the CDPH mandate to include reporting of all CROs and requires isolate submission when the carbapenemase-producing status is unknown or only confirmed through phenotypic testing. This Order will allow ACPHD to close this gap in surveillance and help prevent transmission of CROs in our dynamic patient population.
With 12 acute care hospitals, 1 long-term acute care hospital, and over 70 skilled nursing facilities (SNFs), Alameda County contains a complex healthcare system which services a population of over 1.6 million people. Interconnected facilities and patient sharing between local health jurisdictions create greater vulnerability for transmission and complex outbreaks as compared to more isolated healthcare systems. Reporting of CROs and sequencing of the associated isolates provides crucial, necessary information required to control the spread of CROs in Alameda County. Provider reporting of CRO cases provides valuable information on patient demographics and SNF residential status which has helped ACPHD detect and respond to CRO outbreaks earlier and more efficiently in our dynamic patient population. Provider reporting specifically helps ACPHD identify and interrupt chains of transmission between healthcare facilities and provides information on patients who may be moving between different SNFs and acute care hospitals. Sequencing associated isolates has helped ACPHD identify and interrupt chains of transmission. Using this information, ACPHD provides guidance and support to our healthcare facilities, including best practices for transferring, discharging, and cohorting patients who are colonized with a CRO. ACPHD’s Health Officer Order goes beyond CDPH’s requirement of laboratories reporting only CPOs in order to support our local healthcare facilities that may not have the infrastructure or capacity to test for carbapenemase genes. By requiring the submission of all CRO isolates with unknown carbapenemase-producing status, ACPHD is filling in a known gap in infection prevention and control and working to limit the public health impact of these pathogens.
If you have a CRO outbreak
Important documents
- ACPHD CRE Recommendation for Long-Term Care Facilities
- ACPHD CRE Recommendations for Acute Care Facilities
- ACPHD Public Health Laboratory Specimen Submittal Instructions
- ACPHD Public Health Laboratory Requisition Form for Bacterial Isolates
- ACPHD Interfacility Infection Control Transfer Form

